Clinical Trials Registry
Research
Clinical Trials Registry
Clinical trials hold enormous potential for benefiting patients, improving therapeutic regimens and ensuring advancement in medical practice that is evidence based. Unfortunately, the data and reports of various trials are often difficult to find and in some cases do not even exist as many trials are abandoned or are not published due to “negative” or equivocal results. However, this tendency for availability of only selective information from the myriad clinical trials conducted is not commensurate with the practice of “evidence-based medicine”.
Registration of all clinical trials conducted in a centralized clinical trials registry will ensure transparency, accountability and accessibility in order to re-establish public trust in clinical trial data.
Benefits of Clinical Trials Registration
1. Improve transparency and accountability: By disclosing all required details of the protocol of trials, public confidence in clinical trials is likely to be enhanced.
2. Improve the internal validity of trials: Disclosure of the method of random sequence generation, adequate concealment of allocation of participants to interventions, adequate blinding of participants, investigators and outcome assessors and inclusion of all participants’ results will be mandatory during restoration of trial. These methods of the trial are particularly important to produce reliable results by minimizing biases, confounders and the effects of chance or coincidence. Incorporating such elements at the protocol stage is likely to increase the internal validity of the trial and also increase the chances of publication in a high impact journal.
3. Conform to accepted ethical standards:
i. It will help give ethics review boards considering approval of new studies a view of similar work and data relevant to the research they are considering.
ii. Clearance by local ethics committees is mandatory for all clinical trials for registration.
4. Reporting of all relevant results of registered trials: All the trials registered will be adequately reported and publicly available.
i. Clinical trial registration will prevent selective publication and selective reporting of outcomes.
ii. It will prevent unnecessary duplication of research effort
iii. Describing clinical trials in progress can make it easier to identify gaps in clinical trials research
iv. Making researchers and potential participants aware of recruiting trials may facilitate recruitment
v. Enabling researchers and health care practitioners to identify trials in which they may have an interest could result in more effective collaboration among researchers. The type of collaboration may include prospective meta-analysis vi. It will help patients and the public know what trials are planned or ongoing into which they might want to enroll.
5. Consideration for publication: Editors of Biomedical Journals of 11 major journals of India and International Committee for Medical Journals Editors (ICMJE) member journals now require, as a consideration for publication, registration in a public trials registration. Trials must register at or before the onset of patient enrollment.
General Guidelines
- To assist researchers, WHO maintains a list of registries that meet these criteria (http://www.who.int/ictrp/network/primary/en/index.html). Currently 16 registries are listed.
- The Clinical Trials Registry – India (CTRI), set up at the National Institute of Medical Statistics, ICMR, New Delhi is a free and online system for registration all clinical trials being conducted in India (www.ctri.nic.in). Registration of clinical trials in the CTRI is now mandatory, as per notification of the Drugs Controller General (India) (DCGI).
- www.ctri.nic.in)
- Trial registration involves public declaration and identification of trial investigators, sponsors, interventions, patient population etc. before the enrollment of the first patient.
- When registering their protocol, researchers will be asked to provide information such as descriptions of the intervention(s) and comparison(s) studied, study hypotheses, primary and secondary outcomes, eligibility criteria, sample size, blinding, funding, principal investigators, and dates of commencement and anticipated completion of the study.
- It is common for trial registries to review the information for completeness and clarity, so some editing might be needed. The registry will then provide a unique trial registration number to the researchers. This number should be included in all reports of the trial’s results as a link to the registered protocol for editors, reviewers, and readers.
- Submission of Ethics approval is essential for trial registration in the CTRI.
- Multi-country trials, where India is a participating country, which have been registered in an international registry, are also expected to be registered in the CTRI
- Prospective registration can be done any time before the first participant is recruited. Many researchers wait until immediately before recruitment starts, so that any late changes to the protocol (such as alterations requested by an ethics committee) do not necessitate an amendment to the registry entry.
- Although not ideal, protocol amendments are sometimes made after recruitment starts. These should be updated on the registered protocol as well.
- After a trial is registered, trialists are expected to regularly update the trial status or other aspects as the case may be.
- All updates and changes will be recorded and available for public display.
Which studies need to register
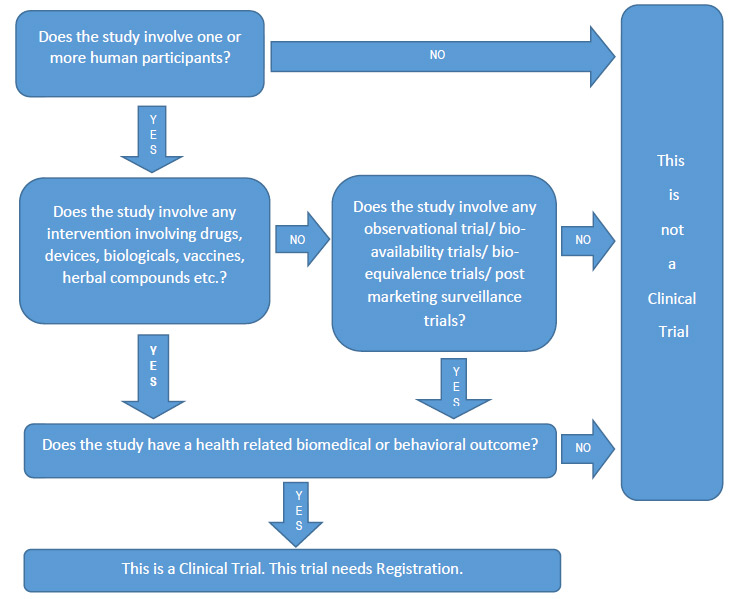
- Studies that meet the WHO/ ICMJE 2008 definition of a clinical trial should be registered.
- For researchers in doubt whether or not to register their trials, both the WHO and ICMJE urges researchers to go ahead and register the trial.
How to register
- Trials should be registered before enrolment of the first patient.
- A Responsible Registrant, Trialist or User is authorized to register clinical trials in the CTRI. Registration in the CTRI is needed to gain access to this facility. The “Responsible Registrant” for a trial is either the principal investigator (PI) or the primary sponsor, to be decided by an agreement between the parties. The primary sponsor is ultimately accountable for ensuring that the trial is properly registered. For multi-center and multi-sponsor trials, it is the lead PI or lead sponsor who should take responsibility for registration.
- To register their clinical trials, trialists must first register as users (obtain username and password). The username and password may be obtained by accessing the Home Page of the CTRI (www.ctri.nic.in) and filling the NEW USER form online and submitting it online. A confirmatory mail will be sent to the email ID provided and upon acceptance of this, an automated mail will be sent to inform the bona fide Registrant of the username and password.
- Upon receiving username and password, the Registrant may login to the CTRI site and then click on TRIAL REGISTRATION and proceed to fill the Trial Registration Form. The form is available in several Parts. After filling Part 1, the data set form may be filled at the convenience of the Registrant. A trial may be submitted only after all the Parts of the data set are completed. Once the options “Approved/No Objection Certificate” (for EC approval status) or “Obtained/notified” status (for DCGI approval) is selected, the Registrant must upload the relevant documents to be able to SUBMIT the trial for further necessary action. Unless the SUBMIT button is clicked, the trial is not visible to the CTRI administrator.
- Once a trial is submitted to the CTRI, the CTRI scientists review the trial data set for meaningful and relevant entries. EC/DCGI approval documents are checked and verification mails sent to trial PIs and Contact persons. In case of any discrepancies or concerns, the trial may be sent back to the Registrant for appropriate modifications and/or clarifications. Upon satisfaction of the above criteria, the trial is registered and trial details viewable from the public domain. The uploaded EC/DCGI approval documents are not available in the public domain.
- A Registrant is expected to regularly update the trial status and other details of a registered trial (as applicable) in a timely manner. While the “Status of Trial” and sites with EC
- approval which are “Under Review” may be updated at any point of time after trial registration, other data set fields are “locked” upon registration. These fields may be “unlocked” after appropriate communication with CTRI scientists and then edited.
- There is no charge for registering a trial. Registered trials are also freely accessible to the public.
- The Universal Trial Number (UTN) (earlier known as the UTRN) is a unique number which aims to facilitate the unambiguous identification of clinical trials registered in WHO Primary Registries and displayed on the WHO International Clinical Trials Registry Platform’s (ICTRP) Search Portal. It is not a registration number. A UTN should be obtained early in the history of the trial. UTN may be obtained from apps.who.int/trialsearch/utn.aspx. Once obtained UTN number must be quoted under SECONDARY ID. Currently obtaining the UTN is not mandatory.



